Magnesium and CO2 Reaction
A demonstration of the reaction between magnesium and carbon dioxide produces magnesium oxide and carbon. Comparison to combustion in air and a pure oxygen experiment.
00:00:03 A demonstration of burning magnesium ribbon in a CO2 atmosphere shows a reaction that produces magnesium oxide and free carbon as a soot. CO2 is reduced and magnesium is oxidized.
🔥 Burning magnesium ribbon in a CO2 atmosphere results in a reaction where magnesium is oxidized and CO2 is reduced.
⚪️ The reaction produces magnesium oxide and free carbon (soot).
⚫️ Squeezing the burning magnesium against the beaker wall leaves a black carbon stain.
00:01:14 A demonstration of the reaction between magnesium and CO2 compared to combustion in air, with an additional experiment using pure oxygen.
🔥 The reaction of magnesium with CO2 produces carbon residues.
⚪ Combustion of magnesium in air produces a whiter ash.
✨ Burning magnesium with pure oxygen increases the brightness of the reaction.
You might also like...
Read more on Science & Technology
NC State University - College of Natural Resources - Environmental Sciences Major

Wörter und Sprachen - Automaten und formale Sprachen 1
![[Why series] Earth Science Episode 3 - High Air Pressure and Low Air Pressure](https://i.ytimg.com/vi/DquXO2FEl0Q/maxresdefault.jpg)
[Why series] Earth Science Episode 3 - High Air Pressure and Low Air Pressure

Texmaker Tutorial: How to Install and Get Started on Windows

Point culture - Les sports en France
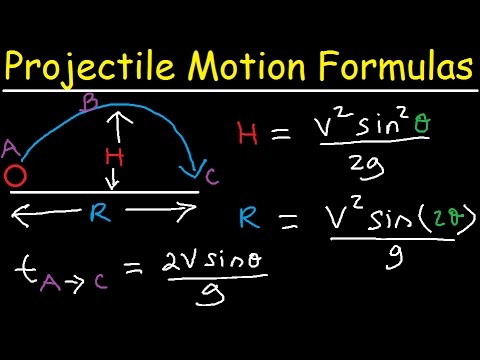
Introduction to Projectile Motion - Formulas and Equations