Understanding covalent bonding between atoms for stability.
Formation of stable molecules through electron sharing.
00:00:27 Covalent Bonding: Formation of stable molecules through electron sharing.
💡 Noble gases have complete outer electron shells, making them stable.
💡 Covalent bonding occurs when atoms share electron pairs to achieve stability.
💡 Covalent bonds are formed through the sharing of electron pairs between atoms.
00:01:56 Understanding covalent bonding between chlorine atoms to achieve stability.
🔑 Covalent bonding is formed when two atoms share electrons to achieve stability.
💡 Chlorine atoms have seven electrons in their valence shell and need one more electron to achieve stability.
👥💻 When two chlorine atoms come together, they share electrons to form a chlorine molecule, resulting in each atom having eight electrons in their valence shell.
00:03:16 Understanding covalent bonding and electron sharing between atoms for stability.
🔗 Covalent bonding is a type of chemical bond formed by the sharing of electrons between atoms.
💡 In a covalent bond, atoms share electrons in order to achieve a stable electron configuration.
🌐 By sharing electrons, atoms can become more stable and form molecules.
00:04:26 When two oxygen atoms come together, they share two pairs of electrons to form a double bond. Similarly, nitrogen atoms share three pairs of electrons to form a triple bond.
🧪 When two oxygen atoms come together, they form a double bond by sharing two pairs of electrons.
⚛️ Similarly, nitrogen atoms form a triple covalent bond to create a nitrogen molecule.
You might also like...
Read more on Education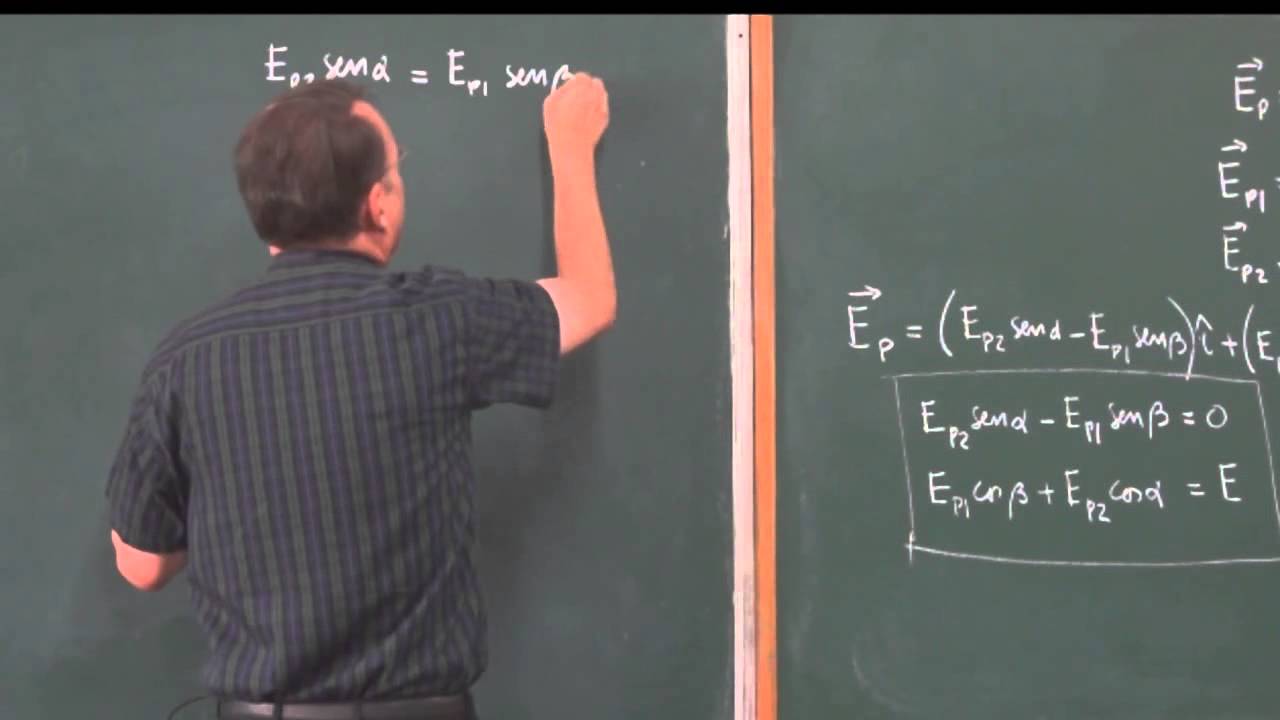
Clase 12: Problema 5; Cálculo de la magnitud del campo eléctrico debido a dos cargas.

8 Tipos de Personas que Nadie Debe Ayudar según los Principios Estoicos

Modulo 7 Intervention en crisis
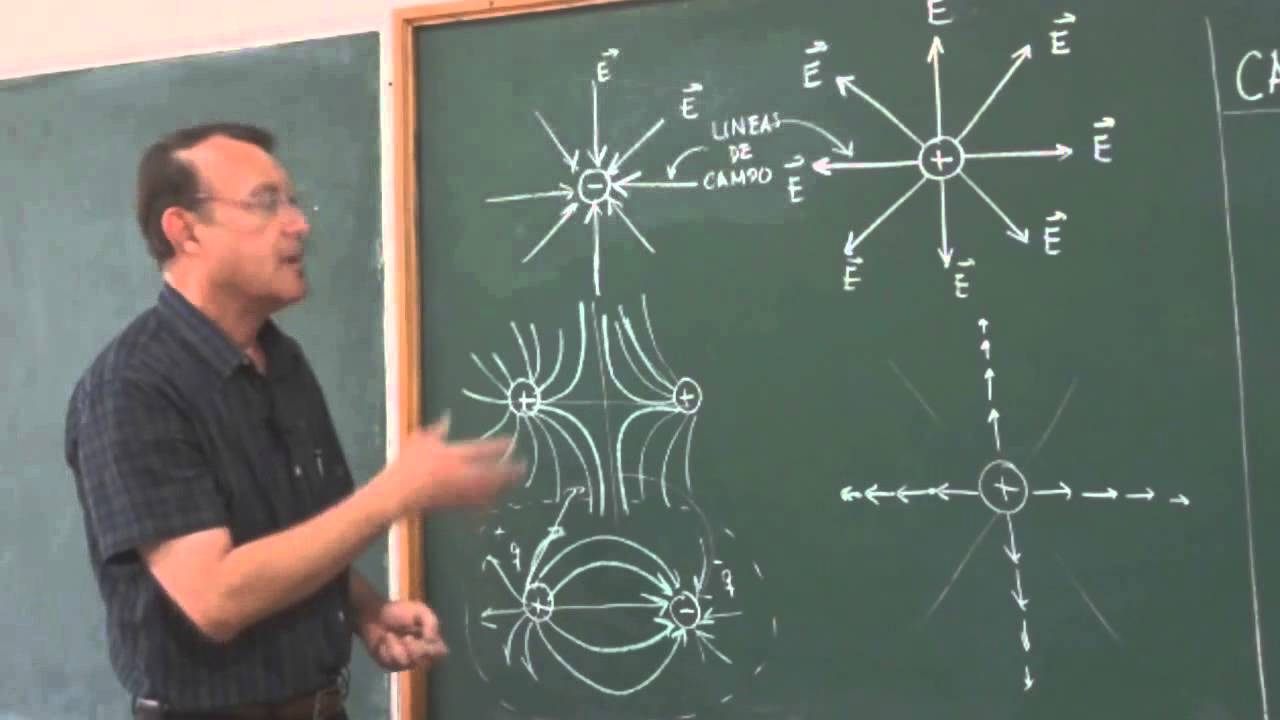
Clase 11: Concepto del Campo Eléctrico para una carga puntual y un conjunto discreto.

Manipulación y el poder de las emociones | DW Documental

What Were the Townshend Acts? | History