Understanding the Redox Reaction Between Zinc and Hydrochloric Acid
Learn about a redox reaction between zinc and hydrochloric acid, explaining reduction, oxidation, and exothermic reactions.
00:00:01 A simple redox reaction occurs when zinc reacts with hydrochloric acid, releasing hydrogen gas. Safety precautions must be taken due to the irritating vapors.
🔬 The video demonstrates a simple redox reaction between zinc and hydrochloric acid.
⚠️ Safety precautions must be taken when working with hydrochloric acid, including wearing gloves and protective goggles.
💥 During the reaction, hydrogen gas is released, which can be ignited.
00:01:08 Chemical reaction between zinc and hydrochloric acid. Redox displacement reaction that is exothermic.
⚗️ The reaction between zinc and hydrochloric acid is a redox displacement reaction.
🔥 The reaction is exothermic and releases heat.
🧪 Zinc oxidizes and loses 2 electrons, while hydrogen reduces and gains the electrons.
00:02:12 A chemical reaction between Zinc and Hydrochloric Acid is explained, highlighting the concepts of reduction, oxidation, and exothermic reactions.
🔬 The video discusses the reaction of Zinc with Hydrochloric Acid and explains it as a redox displacement reaction.
⚡ There is a mnemonic rule mentioned, 'drop, gain, reduce, oxidize', which helps to understand the oxidation and reduction process.
🔥 The reaction between Zinc and Hydrochloric Acid is exothermic, meaning it releases heat.
You might also like...
Read more on Education
Time Matters: Teacher Collaboration for Learning and Leading
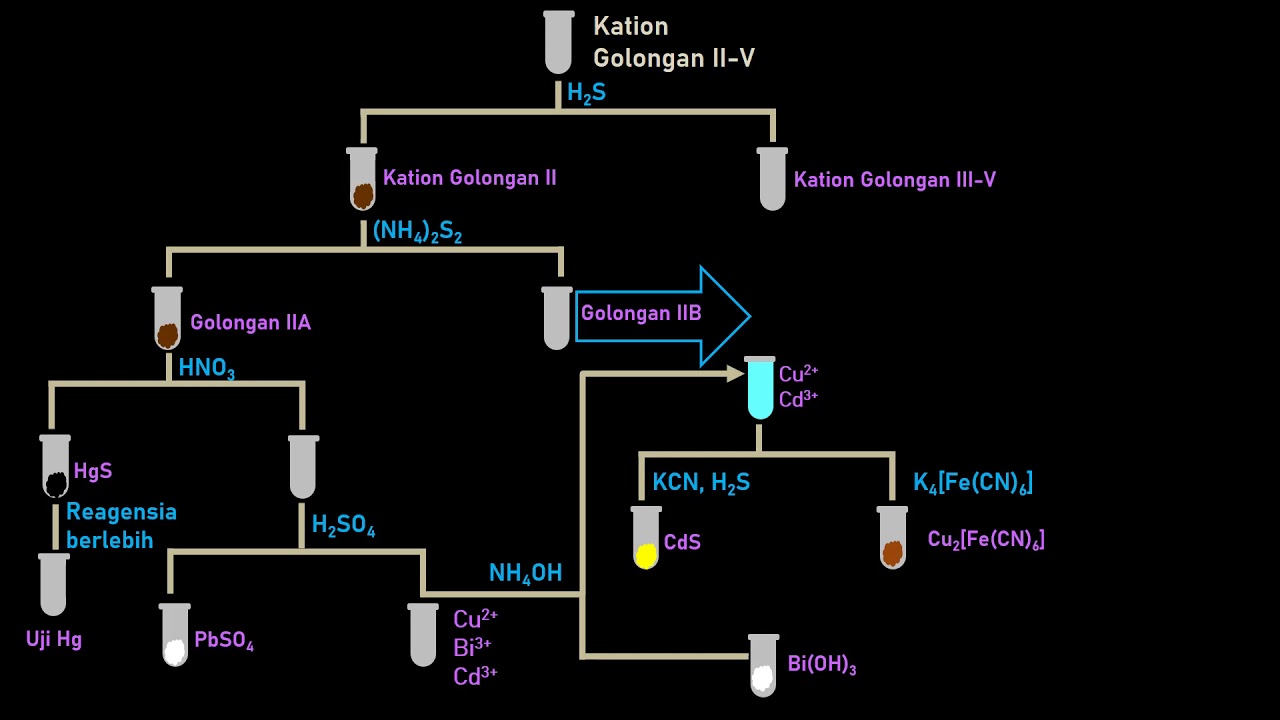
2. Analisis Kualititatif Kation dan Anion

Objeto y método de la Ciencia Política | Educatina

The Horrific Downfall Of Ronaldo Nazario

Empati 35. Bölüm - Kenan İmirzalıoğlu
Data analytics Project on Instagram user analysis ||Driving Engagement|| MYSQL workbench